[dkpdf-columns columns=”3″ equal-columns=”false” gap=”10″]
Introduction
Diabetes Mellitus is a metabolic disorder characterised by impairment of the body’s ability to handle glucose. This occurs as a result of a relative or absolute lack of insulin, resulting in a state of hyperglycemia (or high blood sugar).
There is an expected 55% increase in the global prevalence of diabetes by 2035. Africa is projectedto have the highest percentage increase in prevalence, ahead of all other regions of the world, with an estimated 110% increase by 2035[1].
The World Health Organization estimates that by 2030, there will be 18,234,000 diabetic cases in Africa, of which 4,835,000 will reside in Nigeria alone[2]. Diabetic retinopathy, acceleration of cataracts, glaucoma, anterior ischaemic optic neuropathy, diabetic papillopathy, and neuropathy involving the 3rd, 4th or 6th cranial nerves are some of the main ocular complications of diabetes.Diabetes is best known for its microvasculopathy or effects on the small arteries and blood vessels(microcirculation) of organs, notably the kidney, eyes, neurons and brain among others.
Diabetic Retinopathy (DR) is the most common ocular microvascular complication of type 2 diabetes. Risk factors for DR include long duration of diabetes, poor or unsatisfactory glycemic control, hypertension, anaemia, hyperlipidemia, nephropathy, pregnancy, smoking and obesity. Diabetic retinopathy is an insidious disease and often manifests in the form of vision loss arising from diabetic macula oedema (exudative change), which is the commonest cause of vision loss overall. Ischaemia-induced vision loss will also result in proliferative complications, which include bleeding into the vitreous humour (vitreous haemorrhage), tractional retinal detachment (causing pulling ‘stress’ on the macula), and severe ischemia involving the macula. Ischemia can also be severe enough to cause abnormal new vessel formation or rubeosis, evolving into a state of rubeotic or neovascular glaucoma.
The macula is the functional centre of the light-sensitive retina, responsible for sharp, highresolution colour vision. Located within the macula is a tiny ‘pit’ called the fovea centralis, at which the layers of the retina are neatly parted to allow rays of light gain direct access to photo-sensitive receptor cells (cones), thereby creating the clearest vision possible for the individual in bright light. Damage to this important part of the retina results in visual impairment and distortion of vision.
The macula is the functional centre of the light-sensitive retina, responsible for sharp, highresolution colour vision. Located within the macula is a tiny ‘pit’ called the fovea centralis, at which the layers of the retina are neatly parted to allow rays of light gain direct access to photo-sensitive receptor cells (cones), thereby creating the clearest vision possible for the individual in bright light. Damage to this important part of the retina results in visual impairment and distortion of vision.
Pathogenesis
The origin and development these microvascular complications can be explained by the following
3 proposed theories.
-
- Hyperglycemia, a state of elevated blood glucose altering the expression of genes and leading to increased or decreased amounts of certain gene products capable of altering cellular function.
- Glycosylation of proteins giving rise to a series of reactions, leading to considerable alteration of proteins.
- Chronic hyperglycemia resulting in oxidative stress in cells, leading to the formation of an excess of toxic end products of oxidation, including peroxides, nitric oxide and oxygen free radicals.
Histologically, loss of hugely important supporting cells called pericytes is known to be responsible for the damage caused to vascular endothelial cells observed in the eyes of many diabetic patients. Pericytes are irregularly arranged contractile cells that wrap themselves around the retinal capillary endothelial cells and in the walls of capillaries and tiny venules in other parts of the body.
Damage to these cells (and the consequent destruction of retinal endothelial cells) leads almost inevitably to a picture of dilated capillaries and venules (microaneurysms) and breakdown of the all-important blood-retinal barrier, normally responsible for regulating the flow of nutrients and metabolic waste products in and out of a healthy retina
These microaneurysmal dilatations are a hallmark of the process described above, the end result of which is haemorrhage from small vessels and leakage of extracellular fluid, often resulting in macula oedema and deposition of lipoproteins (hard exudates) in the retinal layers, visible on close examination of the eye as distinct white or yellowish deposits with sharp margins and varying in size from small specks to much larger patches.
On another hand, microvascular occlusion may occur as a consequence of haematological and vascular abnormalities, resulting in retinal ischaemia with cotton wool spots, Intraretinal Microvascular Abnormalities (IRMA) and neovascularization being the features. An ischaemic retina produces vascular endothelial growth factor (VEGF) which stimulates new vessel growth, hence use of the term proliferative diabetic retinopathy (PDR).
The consequence of an oedematous or ischaemic retina is the loss of function resulting in vision loss, assuming involvement (or damage) of the central retina or macula.
New vessels are prone to bleeding (vitreous haemorrhage) and the accompanying fibrosis leads to tractional retinal detachment. Thus, the sight-threatening manifestations of DR are proliferative retinopathy and diabetic maculopathy, which are both preventable and treatable before vision is lost. The risks of development and progression of retinopathy are related to factors such as glycemic control (or HbA1c), blood pressure and blood lipid levels [3].
Vascular Endothelial
Growth Factor (VEGF)VEGF is a 45-KD glycoprotein, which was discovered in 1993. It plays an important role in the pathogenetic cascade promoting vascular growth and permeability. It has been found to promote vascular leakage as well as angiogenesis (formation of new blood vessels originating from preexisting ones).
An elevated level of circulating VEGF is present in conditions with retinal ischaemia. VEGF165 appears to be the dominant isoform in the pathogenetic pathway (in conjunction with other known isoforms and pro-inflammatory mediators). Therefore, the biologic blockage of VEGF in recent times has become a very effective treatment for this condition as will be discussed in the treatment section of this article.
Clinical Presentation
of Diabetic RetinopathyDiabetic retinopathy may present initially with vision loss due the processes explained above. Several cases can also be detected before vision loss occurs by identification, on fundus examination, of the various exudative, non-proliferative and proliferative features of the disease described earlier in this article. Oftentimes, an eye may manifest a combination of exudative, non-proliferative and proliferative features. In such cases, the various features of diabetic retinopathy as enumerated in the pathogenesis section of this article can nonetheless be observed.
Investigations
Ocular-specific investigations are usually required aside from tests needed for systemic evaluation of the patient. The value of such ocular investigations includes assessing the current extent of the disease, making a treatment plan and monitoring the outcome of treatment. Necessary investigations are as follows:
- Fundus Photography
- Fundus Fluorescein Angiography
- Ultrasonography and
- Optical Coherence Tomography
Diabetic Retinopathy –Diabetic Macula Oedema (DMO) & Proliferative Diabetic Retinopathy (PDR)
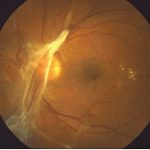
DMO is the most prevalent cause of visual impairment in patients with diabetes. About 7% of diabetic patients have DMO of which 39% have associated visual impairment [4,5]. The typical characteristics of patients with DMO include long duration of diabetes, poor glycemic control, hypertension and hypercholesterolaemia [6,7,8]. Although DMO is often seen in association with non-proliferative diabetic retinopathy (NPDR), it can also be seen in association with proliferative diabetic retinopathy(PDR) (fig 1).
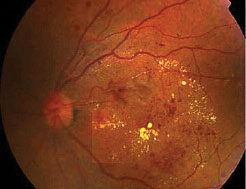
Fig 1: Macula oedema with microaneurysms, hard exudates and haemorrhages. 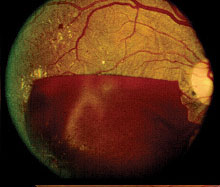
Fig 2: Proliferative disease with large pre-retinal haemorrhage obscuring the macula. Visual acuity is Features of advanced NPDR with a high risk of imminent transformation to PDR include extensive retinal haemorrhages, microaneurysms, cotton wool spots, intraretinal microvascular anomalies (IRMA), and venous beading (fig 2).
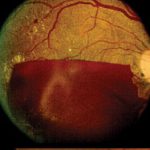
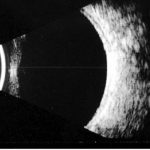
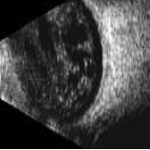
Features of PDR with high-risk characteristics include new vessels on the disc (NVD) >1/3-disc area or new vessels elsewhere (NVE) > 1/2-disc area or vitreous haemorrhage. Mostly, findings on ophthalmoscopy include the clinical features listed above and may include fibrous proliferation. Often, significant pre-retina haemorrhage may be present and result in vitreous haemorrhage. In some cases, significant vitreous haemorrhage may cause vision loss and impair the view of the fundus on clinical examination. An ultrasound B scan can then be a useful tool in assessing the presence and extent of underlying retinal detachment due to vitreoretinal traction. This is illustrated in the figure below, showing a normal B scan and a B scan with significan techogenic vitreous due to a vitreous haemorrhage (fig 3,4).
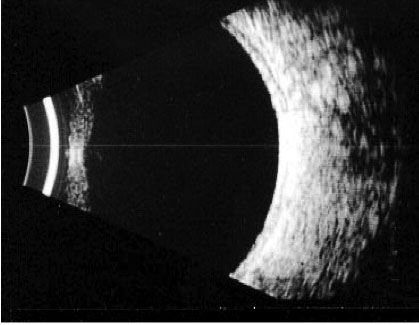
Fig 3: A normal B scan with an echo free vitreous cavity 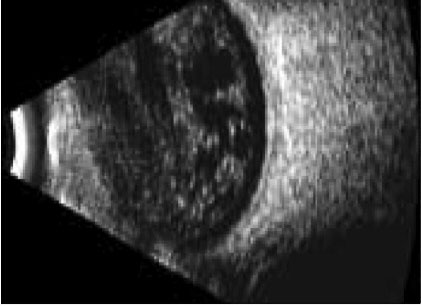
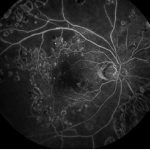
Fig 4: An echogenic vitreous is seen in this B scan. This is indicative of a vitreous haemorrhage A potent insidious cause of loss of vision is tractional retinal detachment, which may commence in an extra-foveal site and then slowly involve the fovea, resulting in significant vision loss. In conditions of clear fundal view, a fundus fluorescein angiography can easily be obtained. Figure 5 illustrates areas of significant retinal and foveal ischaemia. Rubeosis and neovascular glaucoma is a severe presentation characterised by the development of neovascular proliferation within the anterior chamber (including the iris and anterior chamber angles) resulting in a severe rise in intraocular pressure, blindness and pain (fig 9). The optical coherence tomogram (OCT) provides a histological view of the diabetic retina. It is specifically used in the evaluation and monitoring of diabetic macula oedema (DMO). The macular thickness can be determined, including the presence of any traction in the macula.
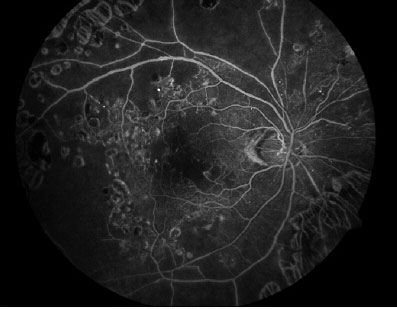
Fig 5: Foveal Ischemia – Retinal Ischemia 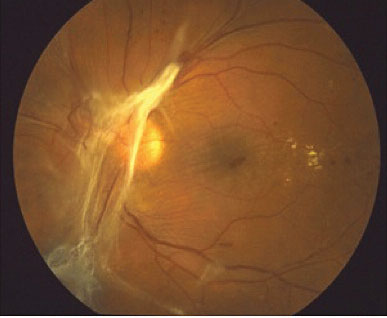
Fig 6: Features of PDR with retinal fibrosis on the retina and tractional retina detachment. Hard exudates are present temporal to the macula. 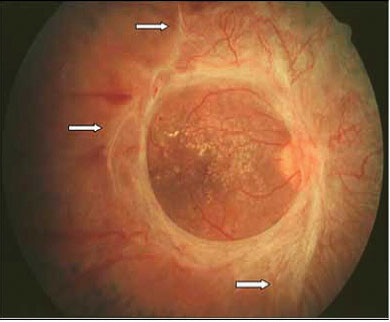
Fig 7: The 3 short white arrows indicate areas of vitreoretinal traction band and associating tractional retinal detachment. 
Fig 8: In clinical situation of pre-retina fibrosis, the fibrous tissue and retinal traction can be assessed using an ultrasound B scan 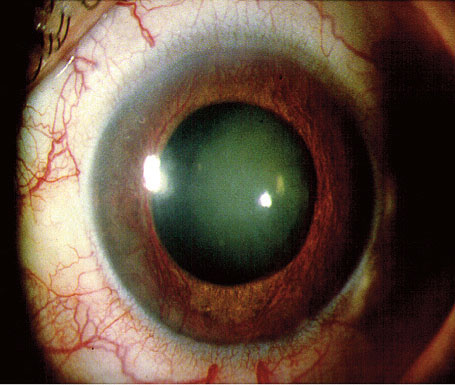
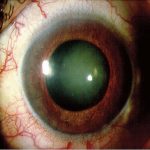
Fig 9: Blood vessels are seen growing on the usually avascular surface of the iris. This is known as rubeosis and results in rubeotic glaucoma with devastating consequences for the eye. Diabetic Retinopathy versus Diabetic Nephropathy
Diabetic retinopathy is useful in diagnosing or screening for diabetic nephropathy in patients with type 2 diabetes and renal disease.
The veracity of this statement is hinged upon the concept and definition of the condition known as microalbuminuria, a small or moderate increase in the level of albumin excretion in the urine confirmed by an albumin/creatinine (ACR) ratio of 3.5 in females and 2.5 in males[10].
Proliferative diabetic retinopathy may be a highly specific indicator for diabetic nephropathy as both are microvasculopathy-related. In one study of 1,414 subjects with type 2 diabetes[9], individuals with macroalbuminuria showed a greater prevalence of DR (60.5% vs. 31.0% vs. 14.1%, p < 0.001) and also a greater severity of the disease (60.9% vs. 21.4 vs. 9.9, p < 0.001) compared with individuals with micro- or normoalbuminuria. Timely evaluation of a patient's renal status is therefore recommended in the event of ophthalmologic detection of the presence of DR or PDR.
Prevention of Diabetic Retinopathy
Prospective controlled interventional studies have shown that strict control of blood glucose and blood pressure significantly reduces and delays the onset and severity of diabetic retinopathy.
Evidence suggests that systematic screening for diabetic retinopathy is cost-effective in terms of sight years preserved compared with the prospect of no screening. Digital photography with telemedicine links has the potential to deliver cost-effective, accessible screening to rural, remote and hard-to-reach populations.
In one study, diabetic patients preferred the teleophthalmology-based screening (stereoscopic digital retinal photographs graded by an ophthalmologist remotely) to the more traditional option of ophthalmologist-based screening for future checking. This preference was driven primarily by convenience, reduced examination time, and ability (of the patient) to visualize their own retina. The use of teleophthalmology in Africa needs further study and has the significant potential to become the screening model of choice. The question of costeffectiveness in comparison to ophthalmologist-based screening also requires further evaluation[11].
Digital photography with telemedicine links, as used by the UK national diabetic eye screening programme has the potential to deliver costeffective, accessible screening to rural and remote populations. Unfortunately, fundus cameras are very expensive. Development of lower-cost solutions could completely change the DR care scenario in Africa. There is a need to develop technologies suitable for the African setting in addition to studies of disease epidemiology and clinical trials focused on detection and management strategies.
So, when do we screen?
Type 1 diabetics: First screen – 5 years after onset/diagnosis and then annually.
Type 2 diabetics: First screen – upon diagnosis and then annually.
Gestational diabetics: As soon as possible after diagnosis.
If diabetes resolves after pregnancy, no further screening is needed.
Treatment of Diabetic Macula Oedema & Proliferative Diabetic Retinopathy
This can be broadly divided into (a) Control of systemic factors; and (b) Available ocular treatments.
- Aim – to prevent development and progression of retinopathy. This includes control of blood glucose, blood pressure and blood lipids.
- Aim – to prevent vision loss and improve vision by the following means:
- Intravitreal Anti-VEGF therapies (injections) – improves visual acuity[12,13]. Examples of commonly used anti-VEGF include Ranibizumab (Lucentis), Bevacizumab (Avastin – off- label use) and Aflibercept (Eylea). All three intravitreal anti-VEGF therapies have demonstrable visual and anatomical benefit in the treatment of DMO.
- Laser photocoagulation treatment – stabilizes vision and reduces the risk of further vision loss, butrarely restores vision[14].
- Intravitreal corticosteroids – commonly given as intravitreal injections or intravitrealimplants of triamcinolone, dexamethasone or fluocinolone. Associated with ocular adverse events such as cataractand glaucoma[15].
- Vitrectomy – surgical technique can be employed in PDR to remove vitreous haemorrhage and fibroticbands responsible for a tractional retinal detachment threatening the macula. Vitrectomy can also be used in cases oftraction-induced macula oedema, where there is a demonstrable thick, taut hyaloid exerting traction on the macula.
Conclusion
Diabetic retinopathy remains a leading cause of blindness amongst diabetics in Nigeria. Broadly-speaking, DMO and PDR are the 2 manifestations of this disease and are both visually damaging when fully established.
The condition is insidiousin onset and early cases may go undetected and have minimal impact on vision until the disease is at an advanced stage. Screening for DR among diabetic patients can result in early detection of the disease, allowing thankfully for early intervention and prevention of blindness.
Diabetic Retinopathy versus Diabetic Nephropathy
Diabetic retinopathy is useful in diagnosing or screening for diabetic nephropathy in patients with type 2 diabetes and renal disease.
The veracity of this statement is hinged upon the concept and definition of the condition known as microalbuminuria, a small or moderate increase in the level of albumin excretion in the urine confirmed by an albumin/creatinine (ACR) ratio of 3.5 in females and 2.5 in males[10].
Proliferative diabetic retinopathy may be a highly specific indicator for diabetic nephropathy as both are microvasculopathy-related. In one study of 1,414 subjects with type 2 diabetes[9], individuals with macroalbuminuria showed a greater prevalence of DR (60.5% vs. 31.0% vs. 14.1%, p < 0.001) and also a greater severity of the disease (60.9% vs. 21.4 vs. 9.9, p < 0.001) compared with individuals with micro- or normoalbuminuria. Timely evaluation of a patient's renal status is therefore recommended in the event of ophthalmologic detection of the presence of DR or PDR.
Prevention of Diabetic Retinopathy
Prospective controlled interventional studies have shown that strict control of blood glucose and blood pressure significantly reduces and delays the onset and severity of diabetic retinopathy.
Evidence suggests that systematic screening for diabetic retinopathy is cost-effective in terms of sight years preserved compared with the prospect of no screening. Digital photography with telemedicine links has the potential to deliver cost-effective, accessible screening to rural, remote and hard-to-reach populations.
In one study, diabetic patients preferred the teleophthalmology-based screening (stereoscopic digital retinal photographs graded by an ophthalmologist remotely) to the more traditional option of ophthalmologist-based screening for future checking. This preference was driven primarily by convenience, reduced examination time, and ability (of the patient) to visualize their own retina. The use of teleophthalmology in Africa needs further study and has the significant potential to become the screening model of choice. The question of costeffectiveness in comparison to ophthalmologist-based screening also requires further evaluation[11].
Digital photography with telemedicine links, as used by the UK national diabetic eye screening programme has the potential to deliver costeffective, accessible screening to rural and remote populations. Unfortunately, fundus cameras are very expensive. Development of lower-cost solutions could completely change the DR care scenario in Africa. There is a need to develop technologies suitable for the African setting in addition to studies of disease epidemiology and clinical trials focused on detection and management strategies.
So, when do we screen?
Type 1 diabetics: First screen – 5 years after onset/diagnosis and then annually.
Type 2 diabetics: First screen – upon diagnosis and then annually.
Gestational diabetics: As soon as possible after diagnosis.
If diabetes resolves after pregnancy, no further screening is needed.
Treatment of Diabetic Macula Oedema & Proliferative Diabetic Retinopathy
This can be broadly divided into (a) Control of systemic factors; and (b) Available ocular treatments.
- Aim – to prevent development and progression of retinopathy. This includes control of blood glucose, blood pressure and blood lipids.
- Aim – to prevent vision loss and improve vision by the following means:
- Intravitreal Anti-VEGF therapies (injections) – improves visual acuity[12,13]. Examples of commonly used anti-VEGF include Ranibizumab (Lucentis), Bevacizumab (Avastin – off- label use) and Aflibercept (Eylea). All three intravitreal anti-VEGF therapies have demonstrable visual and anatomical benefit in the treatment of DMO.
- Laser photocoagulation treatment – stabilizes vision and reduces the risk of further vision loss, butrarely restores vision[14].
- Intravitreal corticosteroids – commonly given as intravitreal injections or intravitrealimplants of triamcinolone, dexamethasone or fluocinolone. Associated with ocular adverse events such as cataractand glaucoma[15].
- Vitrectomy – surgical technique can be employed in PDR to remove vitreous haemorrhage and fibroticbands responsible for a tractional retinal detachment threatening the macula. Vitrectomy can also be used in cases oftraction-induced macula oedema, where there is a demonstrable thick, taut hyaloid exerting traction on the macula.
Conclusion
Diabetic retinopathy remains a leading cause of blindness amongst diabetics in Nigeria. Broadly-speaking, DMO and PDR are the 2 manifestations of this disease and are both visually damaging when fully established.
The condition is insidiousin onset and early cases may go undetected and have minimal impact on vision until the disease is at an advanced stage. Screening for DR among diabetic patients can result in early detection of the disease, allowing thankfully for early intervention and prevention of blindness.
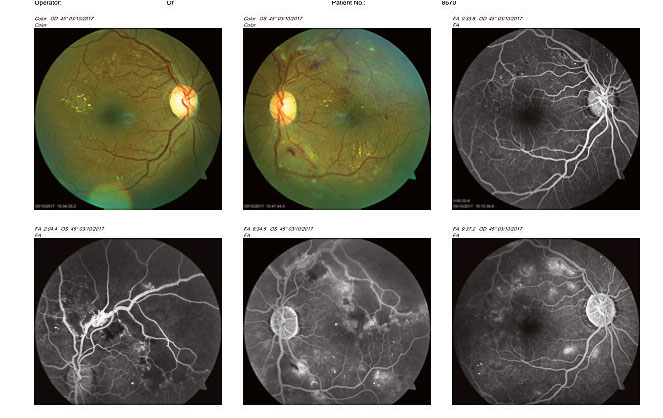
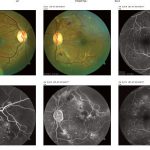
Fig 10: Fundus photograph and fluorescein angiograms showing peri-foveal hard exudates in the right eye macula. 
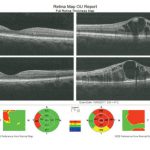
Fig 11: Occipital Coherence Tomogram (OCT) showing dome-shaped macula oedema of the left eye. [/dkpdf-columns]
read more
Disclosure forms provided by the author are available at NEJM.org.
Editor’s note:
Author Affiliations
Supplementary Material
| Disclosure Forms | 83KB |
Add your Comment
Add your Comment
Leave a Reply
You must be logged in to post a comment.
BHQJ 2018 ; 001:34-36
Related Article 
Medical Negligence & the Law5th May 2018 . Atrogenic harm is a matter of significant concern in Nigeria admin
Colorectal Cancer Overview5th May 2018 . [dkpdf-columns columns="3" equal-columns="false" gap="10"] Introduction Colorectal cancer is a major admin
Funding Healthcare Services in Nigeria – A conundrum of demand, policy and supply!5th May 2018 . [dkpdf-columns columns="3" equal-columns="false" gap="10"] Doctor, I happy say na you admin
5 “Provocations” of Healthcare Quality Reform6th May 2018 . [dkpdf-columns columns="3" equal-columns="false" gap="10"] n the four decades since he admin
Health Insurance, Activism & Urgent Change6th May 2018 . [dkpdf-columns columns="3" equal-columns="false" gap="10"] AR: Dr Soyinka, it’s wonderful to admin
Anne Olowu talks about her “Masterclass” experience6th May 2018 . [dkpdf-columns columns="3" equal-columns="false" gap="10"] As I suspect is the case admin
Stomach & Oesophageal Cancer in Nigeria6th May 2018 . [dkpdf-columns columns="3" equal-columns="false" gap="10"] Gastric and Oesophageal (Upper GI) cancers admin
Setting out the Stall!7th May 2018 . [dkpdf-columns columns="3" equal-columns="false" gap="10"] "The drawbacks of our false knowledge admin





Leave a Reply
You must be logged in to post a comment.